Definition of water
Water is a transparent, odourless, tasteless, and nearly colourless inorganic chemical compound made of hydrogen and oxygen (H₂O). It is the most essential substance on Earth, covering about 71% of the planet’s surface and forming the main part of the Earth’s hydrosphere. Water is also a major component in all living organisms, making up 60–70% of the human body.
Even though water doesn’t contain calories or natural nutrients, it is vital for all known forms of life. It helps in digestion, temperature regulation, nutrient transport, and countless other biological and chemical processes.
Structure of the Water Molecule
Water Chemical Formula:

Water Structural formula:
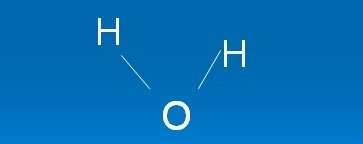
How is a Water Molecule Polar?
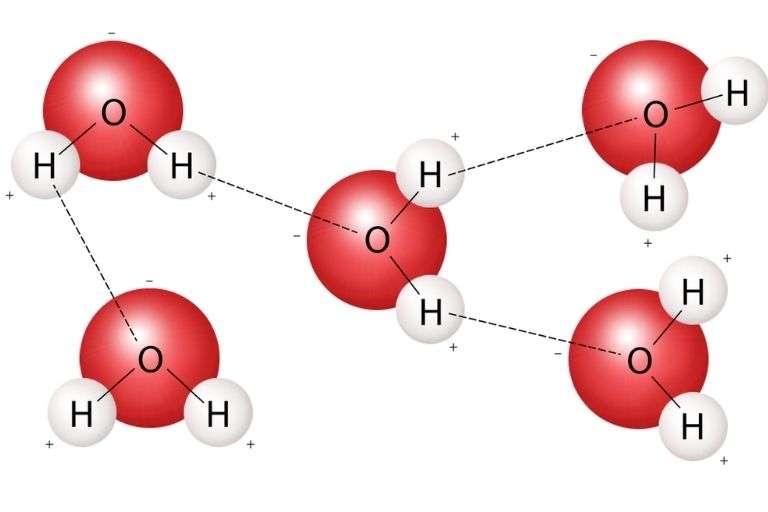
An oxygen atom forms a polar covalent bond between two hydrogen atoms and a water molecule (H2O). Although there is no net charge on a water particle, the polarisation of water creates some positive charges on hydrogen and some negative charges on oxygen, which contribute to the surface tension of water.
Water Molecules Bond With Each Other
Water particles form hydrogen bonds with each other. A partial adverse charge on a molecule O can form a hydrogen bond with a partial positive charge on other particles’ hydrogen. Water particles are similarly brought into other polar molecules and ions.
Read This also: Uses of Water: Importance and Applications
Physical Properties of Water
Three States of Water
Water is the only naturally occurring substance that can exist in all three physical states on the planet.
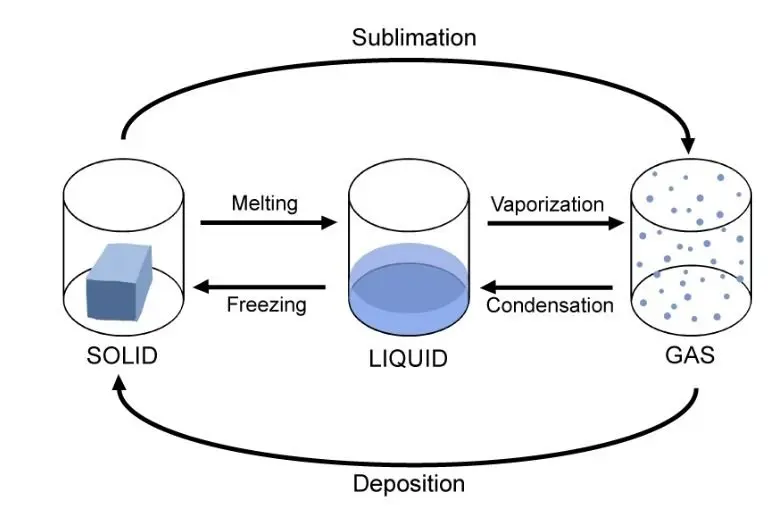
Water Phase Changes
Freezing:

When water freezes it usually goes from liquid to solid. For pure water, the temperature level has to go down to 32 degrees Fahrenheit (0 degrees Celsius) for this. For any compound, the temperature at which it is frozen depends on the energy that holds its particles together.
Melting:
The melting point of water is the temperature at which it changes from solid ice to liquid water. Nevertheless, for useful purposes, the melting point of distilled water ice in a pressure of 1 atmosphere is very nearly 0° C, which is 32 ° F or 273.15 K.
Latent Heat of melting Ice = 334 kJ/kg
This means 334 kilojoules of heat are needed to melt 1 kg of ice at 0°C.

Evaporation:

Evaporation is the method by which water transforms from liquid to gas or vapour. Evaporation is the primary way that water returns from the liquid state to the water cycle as climate water vapour.
Condensation:
Condensation is the method by which water vapour becomes liquid. It is the opposite of evaporation, where liquid water becomes a vapour. Water condenses in two ways: Firstly the air is cooled to its dew point another process it becomes so saturated with water vapour that it can no longer hold water.

Frost Formation:

Frost in the form of water vapour or water gas, which becomes stronger. Frost usually forms on objects such as vehicles, windows, and plants that are filled with air, or filled with moisture. Locations that have a great deal of fog frequently have heavy frosts—types of snowfall when the outer surface area is cold beyond the humidity.
Sublimation:
Sublimation is the conversion of matter into the solid and gaseous phase, without any intermediate liquid phase. Sublimation is often used to describe the process by which ice and ice evaporate in the air without first melting in water.

Water Density
- The density of pure water is 1.0
- The density of pure ice is 0.92
- The density of sea water is 1.03
Most solids are more dense than their liquids. This makes solids sink, but ice is less dense than liquid water due to H-bonds.
Read this also: What is the Density of Water?
What is the water surface tension?
Water molecules stick together and draw inward. This forms a tight layer on the surface. This layer is resistant to being broken apart. This is called surface tension. Surface tension measures the strength of water molecules attracting to one another due to hydrogen bonding.



Surface Tension Effects
- Gravity – Changes the shape of droplets as they fall.
- Warmer temperature – Hot water is a better cleaning agent because the lower surface tension makes it a better “wetting agent” to get into pores and fissures rather than bridging them with surface tension.
- Soaps and detergents break the surface tension.
Read This also: What are the water pollution causes?
Water Specific Heat
Due to the hydrogen bonding, water has the highest specific heat of all natural liquids. Much heat from sunlight is used to break hydrogen bonds.
Water can absorb large amounts of heat and store a lot of heat with slight temperature changes. Ocean currents play a significant role and regulating the environment through thermal conductivity.
The importance of water
Water is the most vital resource on the planet; without it, life can’t exist. But what is it that makes water so special? Water has a variety of properties that make it a helpful resource.
- Water is an excellent solvent. It can dissolve maximum substances.
- Water’s boiling and freezing points make it readily accessible in the three states (solid, liquid and gaseous).
- Water is neither basic nor acidic. It is a neutral material that has a pH of 7.
- The water’s specific heat is very high. So it can able to hold more heat compared to other liquids.




