We can analysis pH of any type of water (Wastewater or Drinking water) in two methods. One method is colourimetric methods and another is electrometric method. In the colourimetric methods, we use chemical reagents to check the water pH and electrometric methods we use an electric meter to analysis of pH. The water pH test is very simple to follow a few steps to do this.
First we are discussing colourimetric pH test procedure for wastewater or drinking water.
pH test procedure ( colourimetric methods )
pH Test with Universal indicator

- Take 10ml test sample, place in test tube and add 2 drops of Universal indicator .
- The display of different colors and you need to match the color with the chart of colors and take note of the correct reading.
Video:
pH Test Paper
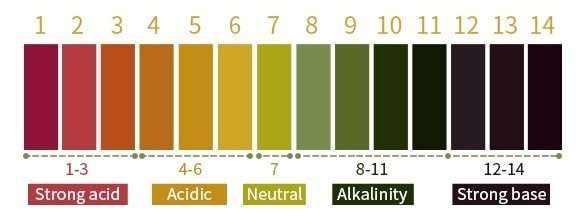

- Simple to use and get the pH result within a second with pH paper. It is an easy way to determine the pH of the solution without costly equipment or pre-calibration, even if you do not have experience in chemistry.
- Deep the pH test paper in the water for 2-3 seconds after comparing the strip’s colour in the pH 1-14 colour chat.
Video:
Related Article: What is pH of Water?
pH test procedure with meter( Electrometric methods)
pH analysis with pH meter is required some technical knowledge of chemistry. you need some apparatus to do this & also need frequent calibration for accuracy of the result.

Apparatus Requirement
- Beaker 200 ml
- pH meter
- Water sample
pH test Procedure
Follow the manufacturer’s guidelines for operating the pH meter. First switch on the pH meter & warming up with standardised with an appropriate buffer solution that is at least pH similar to the sample. Also, check the electrode against at least one more buffer with a different pH value.
When pH analysis is checked in the lab, dip the electrode in the water sample and observe continuous readings. Wait for a stable reading & record your sample and record the pH with the temperature data. Record the pH to the nearest coefficient, or 0.01 unit.
pH Meter Callibration
Create three different ( at least two ) standard solutions comprising of 4, 7, and 9.2 by dissolving the buffer tablet in 100ml deionised drinking water.
The electrode must be submerged in the buffer solution known as pH like 7.0; if reading found some variation, you need to adjust pH 7.0.
The electrode system is separated from buffer solutions, washed using distilled water. Then, it is placed in a different buffer 4.0 pH. The meter reading is adjusted to the 4.0 value through slope adjustment.
The electrode system has been separated from buffer solutions, cleaned with distilled water, and then placed in a new known buffer solution, say 9.2 pH. The indicator of the instrument should show this value, and if it does, it means that the electrode system functions accurately.
Related Article:



Thanks for posting. I really enjoyed reading it, especially because it addressed my problem. It helped me a lot and I hope it will help others too.