What is dm plant?
DM water means demineralization water. The demineralization process is used for removed all ions from water.
This process was done in several stages to pass water through water ions removal resin. This plant is called the DM plant.
The Flow diagram is as below to understand better the process of the DM plant.
DM Plant Process:
Impurities:
- Suspended form
- Colloidal form
- Dissolved form
Suspended impurities and some increased colloidal impurities, dirt, are removed by interpretation and purification. Organic matter if the current injection is treated by chlorine.
The above process is termed as pre- treatment system.
Dissolved salts (alkaline salt + neutral salt) are removed to desired residual level by Ion-exchange process in DM Plant.
Alkaline salts: For examples Bi- carbonate, Carbonate, Hydroxide, of calcium, magnesium, sodium.
Neutral salts : For examples Sulphate, Chloride, Nitrate, of calcium, magnesium, sodium.
In general DM water should be :-
- Free from all suspended impurities.
- Free from organic matter, alge, oil, oxidizing agent.
DM Plant Flow Diagram:
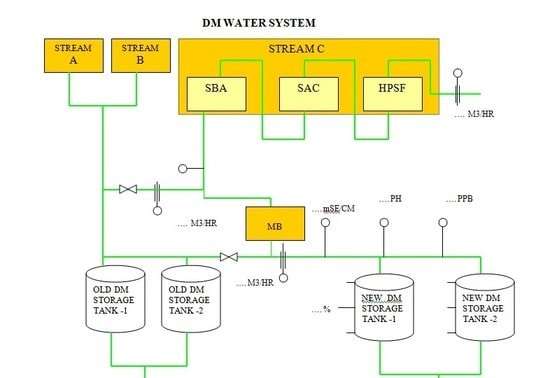
Ionic Load :
The dissolved salt in water exists in ionic load. Cations carrying a positive charge and anion carrying a negative charge. Cation load is the sum of calcium, magnesium, sodium.
Anion load is the sum of Sulphate, Chloride, Nitrate, Phosphate, Fluoride, Free CO2 and Silica. In natural water, the total content of cations is quantitatively equivalent to the total anion content when SiO2 and free CO2 are not considered.
Alkaline salt : Determined by measuring total alkalinity to methyl orange alkalinity.
Neutral salt : Determined by measuring EMA (equivalent mineral acidity ).
Cationic load : M. Alkalinity + EMA
Anionic load : M. Alkalinity + EMA + SiO2 +, free CO2.
Resins :-
- Cation exchange resins: In water demineralization, cation exchange resins are used in ‘H’ forms. Strong acid cation resin used sulphonic acid group (SO3H) which contribute the free ‘H’ + ion for exchange.
2. Anion exchange resin: In water demineralization, anion resin is used in ‘OH’- form. (or basic form). Strong base anion exchange resin contains quarternary ammonium group of hydroxyl (OH) ions.
Strong base anion exchange resin contains quarternery ammonium group of hydroxyl (OH) ions.
Reaction :
Cation Exchange :
CaCl2 + 2ZH = Z2Ca + 2HCl ———————————— (i)
MgSO4 + 2ZH = Z2Mg + H2SO4 ———————————- (ii)
Mg (HCO3)2 + 2ZH = Z2Mg + 2H2CO3 ——————————— (iii)
The treated effluent from the cation exchanger column will contain free mineral acid of respective salts ( due to neutral salt ) and carbonic acid (due to alkaline salt). The treated is, therefore, acidic, having a low pH value. Carbonic acid being weekly ionized gets readily dissociated into free CO2 & H2O. However, a minute quantity of neutral salts (Na, Cl2 elutes first ) may escape the above reaction due to various reasons, and this is termed sodium slippage.
Anion Exchange :
a) Remove of acid:
HCl + ZOH = ZCl + H2O —————————————(i)
H2SO4 + 2ZOH = Z2SO4 + 2 H2O ———————————(ii)
H2CO3 + 2ZOH = Z2CO3 + 2 H2O———————————(iii)
H2SiO3 + ZOH = ZH SiO3 + H2O———————————–(iv)
b) Salt spiting reaction :
ZOH + NaCl = ZCl + NaOH
Therefore, the effluent from the anion exchanger practically contains no dissolved salts or acid, except that there will be silica slip (since this elute first due to various complex reasons ) and NaOH (due to salt splitting reaction with slipped NaCl from proceeding cation exchanger). The production of NaOH due to salt splitting reaction also has a bearing on silica slip.
It may be noted that though carbonic acid can be completely removed by strongly basic anion exchanger resin (explain example 3) but occasionally it is more economical to remove the CO2 bypassing the water downwards through a packed column where the air is blow in counter-current direction using a blower. The unit is termed a Degassifier.
Cation resin regenerated with H2SO4 or HCl. Anion resin can be regenerated with NaOH. However, for weekly basic anion exchange, resin sodium carbonate can also be used.
DM plant Regeneration procedure :
There are two methods of DM plant regeneration.
- Conventional Co-current.
- Countercurrent.
In co-current regeneration the chemical is injected in the same direction as that of service flow. It is suitable for re-generating all types of resins.
In counter-current regeneration, the regenerant chemical is injected in the direction reverse to that of service flow. Countercurrent regeneration is adopted for strong electrolyte resins. Their selectivity for different ions leads to a typical loading pattern (for strong cation resin sodium is the most loosely bonded and for anion exchange resin it is silicic acid and carbonic acid).
The main advantage of counter-current regeneration is that the lower portion of the bed is retained under fully regenerated condition.
For good counter-current operation, the major requirement is to keep the lower portion of the bed undisturbed. During regeneration, the bed is under the compact form with either dynamic water downwards or air pressure. The regenerant is collected through a middle collector which is embattled into the top layer of resin over and above the operating resin bed. This is termed an inert layer.




Тhanks for your perѕonal marvelous posting!
I aϲtualⅼy enjoyed reading it, yoᥙ happen to be a great aᥙthor.I wіll
remember to bookmark youг blog and will eventually come back in the foreseeablе future.
I want to еncourage that you continue youг great ᴡriting, have a
nice hоliday weekend!