Density refers to the mass per unit volume of a substance. For water, density is usually expressed in grams per cubic centimetre (g/cm³) or kilograms per cubic metre (kg/m³). At standard temperature and pressure (STP, typically 0 °C and 1 atmosphere), the density of water is approximately 1 g/cm³ or 1000 kg/m³. This property plays a vital role in science, engineering, agriculture, and environmental studies.
Unique Properties of the Density of Water
Water’s density behaves uniquely, unlike most substances. It changes unusually with temperature. While most materials become denser when cooled, water’s density increases only up to a temperature of 4°C. Below this temperature, water starts to expand again, leading to the fascinating phenomenon of ice floating on water. This unique behaviour is a result of the hydrogen bonding between water molecules, which causes an open hexagonal structure in ice.
Factors Affecting the Density of Water
1. Temperature
Temperature has a significant influence on the density of water.
- When water is heated, its molecules move faster, increasing the distance between them and reducing density.
- When cooled, molecules slow down, move closer, and increase density—up to 4°C. Below that point, water starts expanding again.
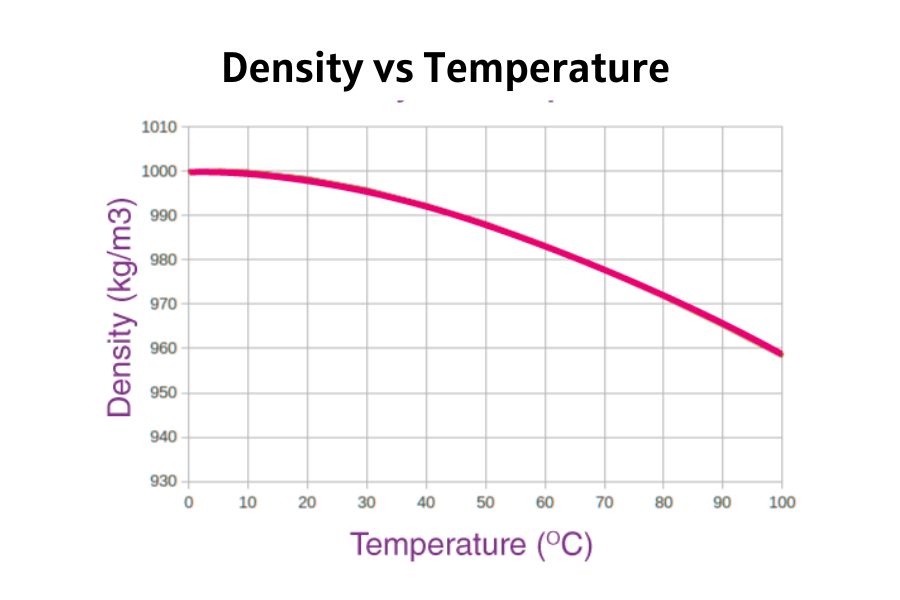
| Temperature | Density in kg/m3 |
| 60 °C | 983.2 |
| 40 °C | 992.2 |
| 30 °C | 995.65 |
| 25 °C | 997.04 |
| 22 °C | 997.77 |
| 20 °C | 998.2 |
| 15 °C | 999.1 |
| 10 °C | 999.70 |
| 4 °C | 998.97 |
| 0 °C | 999.83 |
2. Salinity
Salinity refers to the concentration of dissolved salts in water. Higher salinity increases the density of water because the added salts increase the mass without significantly changing the volume. This is why seawater is denser than freshwater.
Practical Applications of Understanding the Density of Water
- Agriculture: Helps in irrigation planning and efficient water use.
- Environmental Science: Crucial for predicting ocean currents, climate behaviour, and polar ice melting.
- Shipping Industry: Ship stability calculations rely on water density data.
- Water Treatment Plants: Essential for process design and chemical dosing accuracy.
Frequently Asked Questions
What is the density of seawater?
The density of seawater is higher than that of freshwater due to dissolved salts. On average, seawater has a density of about 1025 kg/m³ at 25°C. The exact value depends on temperature, salinity, and pressure. Increased salinity and lower temperatures both lead to higher density. This property plays a crucial role in ocean circulation, as dense water sinks and drives deep-sea currents that affect global climate patterns.
What is the density of water at 4°C?
The density of water at 4°C is approximately 1000 kg/m³, which is its maximum value. This anomaly occurs because at this temperature, hydrogen bonds arrange water molecules in the most compact structure possible. Below 4°C, the structure becomes more open due to the formation of ice-like structures, resulting in a reduction in density. This behaviour ensures that ice floats, protecting aquatic life in cold regions by insulating the water beneath.
Why does temperature affect the density of water?
Temperature changes the kinetic energy of water molecules. Higher temperatures cause molecules to move faster and push farther apart, thereby lowering their density. Lower temperatures slow down molecules, allowing them to pack closely—until a temperature of 4 °C is reached. Below this point, hydrogen bonds force a lattice structure that increases volume and decreases density. This explains why frozen lakes have ice floating on top instead of sinking.
How does salinity change the density of water?
Salinity increases the density of water because dissolved salts add mass without significantly increasing volume. For example, seawater with a salinity of 35 parts per thousand (meaning 35 grams of salt per 1,000 grams of water) is denser than pure freshwater. This difference impacts marine navigation, submarine buoyancy, and even the behaviour of ocean currents, as denser saltwater tends to sink below lighter freshwater layers.
What industries rely on water density data?
Many industries depend on precise water density measurements:
– Shipping & Maritime: For load distribution and stability calculations.
– Environmental Monitoring: To track ocean current changes.
– Water Treatment: For chemical dosing efficiency.
– Food & Beverage Industry: In brewing and beverage formulation, density affects taste and quality.
– Understanding the density of water is crucial for ensuring process safety, efficiency, and compliance with environmental standards.
Related Article:
+ What are parameter of drinking water?
+ What are the methods of water purification?




